Biocatalysis is the use of enzymes or cells to carry out defined chemical reactions under controlled conditions, in order to convert raw materials into commercially more valuable products. The high selectivity of enzymatic transformations, combined with the mild reaction conditions and the use of inexpensive reagents, represent sound advantages of biocatalysis. In order to render the enzymes more suitable for industrial conditions, we must first study how their structure correlates with their function. X-ray crystallography, biophysical and biochemical characterization, as well as site specific mutagenesis allow us to gain insight into the enzymes’ structure and mechanism. Subsequently, we employ protein engineering methods to tailor the biocatalyst for its intended use.
Unraveling the mechanism of tyrosinase: the enzyme that makes us colorful
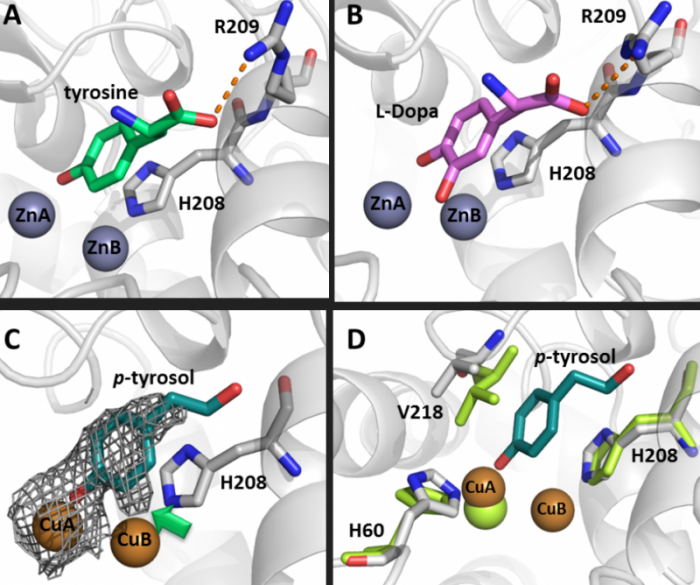
Tyrosinase, is responsible in human melanocyte cells for the first step in the synthesis of the dark pigment melanin. Mutations in this enzyme are the main cause for albinism, a condition that causes impaired vision and sensitivity to light. In fruits and vegetables tyrosinase initiates browning, which results in economic losses to farmers. Our studies revealed that all of the substrates are stabilized in the same orientation at the active site of the enzyme, and are positioned towards the same copper ion. Understanding of the mechanism and structure of tyrosinases now helps us in developing inhibitors for treatment of hyperpigmentation and age-related spots, improving the sensitivity of melanoma cells towards radiation therapy, and preventing browning of fruits and vegetables.

Biocatalysis for tailoring food structure
The increasing preference of vegetarian and vegan diets has led to the development of new and diverse food formulations based on plant proteins with high nutritive value. Industrial side-products such as functional potato proteins (PP) and non-functional corn fractions (zein) are promising sources of protein in food applications while complementing each other in their amino acid composition. We used tyrosinase (TyrBm) for crosslinking the proteins thus forming a novel gel-like texture, potentially leading to a wide variety of non-meat foods such as cheeses, creams, meat replacements, spreads, mousses, and dressings.

From waste to biodiesel
Enzymatic production of biodiesel by a reaction between triglycerides and alcohol, catalyzed by lipases, offers an environmentally-friendly and efficient alternative to the chemically catalyzed process while using low-grade feedstocks. Methanol is utilized frequently as the alcohol in the reaction due to its reactivity and low cost. However, one of the major drawbacks of the enzymatic system is the presence of high methanol concentrations which leads to methanol induced unfolding and inactivation of the biocatalyst. Therefore, a methanol stable lipase is of great interest for the biodiesel industry. We combined protein engineering with immobilization engineering to design a stable biocatalyst for converting slaughterhouse waste chicken oil into biodiesel.